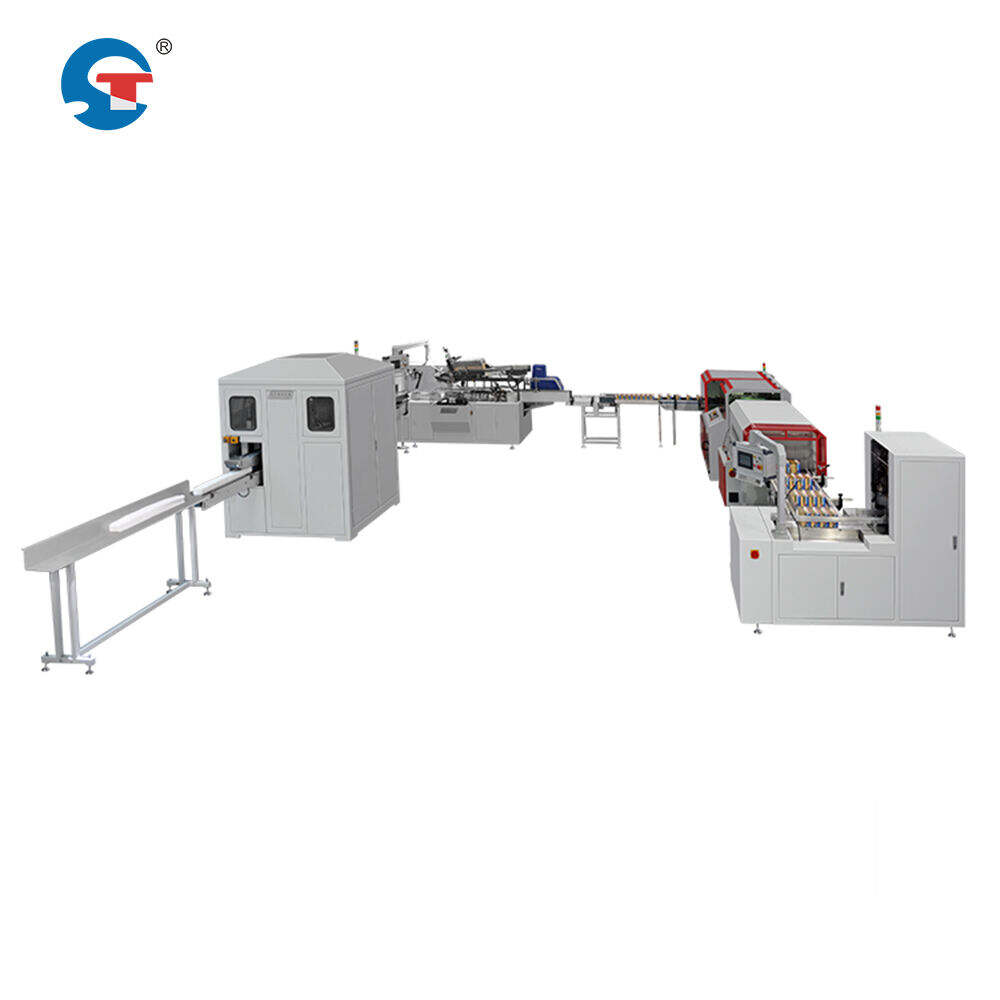
kagamitan sa pag-pack para sa gamot
Ang makinarya para sa pagpapakete ng gamot ay nagsisilbing mahalagang bahagi sa modernong proseso ng pagmamanufaktura at pamamahagi ng droga. Ang mga sopistikadong sistema ay nagbubuklod ng maramihang mga tungkulin upang matiyak na ligtas na napapakete ang mga gamot habang pinapanatili ang kanilang epektibidad at integridad. Sinasaklaw ng makinarya ang iba't ibang yunit, kabilang ang mga sistema ng pagpuno ng bote, mga linya ng blister packaging, kagamitan sa paggawa ng karton, at mga istasyon ng paglalagay ng label. Bawat bahagi ay idinisenyo nang may tumpak na inhinyeriya upang maingat na mapangasiwaan ang delikadong mga produkto sa parmasyutiko. Kasama sa mga sistema ang mga advanced na tampok tulad ng automated na mga sistema ng inspeksyon, mga mekanismo ng tumpak na dosis, at mga hakbang laban sa kontaminasyon. Ang mga makina ay gumagana alinsunod sa mahigpit na mga gabay ng GMP, na may konstruksyon na gawa sa hindi kinakalawang na asero, tugma sa clean room, at mayroong validated na proseso ng pagpapakita. Ang teknolohiya ay nagbibigay-daan sa mabilis na operasyon habang pinapanatili ang katumpakan sa mga operasyon tulad ng pagbibilang, pagpuno, at pag-seal. Ang modernong makinarya para sa pagpapakete ng gamot ay kasama rin ang isinintegradong mga sistema ng kontrol sa kalidad na namamatayag ang mga parameter tulad ng bigat, integridad ng seal, at pagkakaroon ng produkto. Ang sari-saring gamit ng makinarya ay nagpapahintulot dito upang mapangasiwaan ang iba't ibang anyo ng parmasyutiko, kabilang ang mga tablet, kapsula, likido, at pulbos, habang nag-aalok ng mga opsyon para sa iba't ibang materyales at format ng pagpapakete. Ang mga advanced na sistema ng kontrol na may HMI interface ay nagbibigay-daan sa mga operator na subaybayan at i-ayos ang mga parameter nang real-time, upang matiyak ang pare-parehong kalidad ng pagpapakete at kahusayan sa operasyon.